isotope
US /ˈaɪ.sə.toʊp/
UK /ˈaɪ.sə.təʊp/
名詞
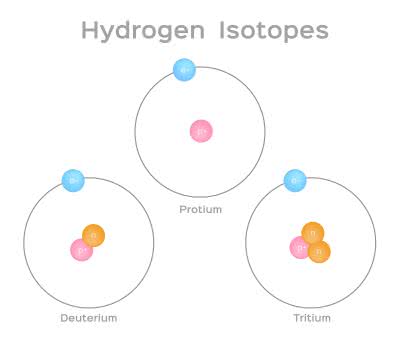
同位体
each of two or more forms of the same element that contain equal numbers of protons but different numbers of neutrons in their nuclei, and hence differ in relative atomic mass but not in chemical properties; in particular, a radioactive form of an element.
例:
•
Carbon-14 is a radioactive isotope of carbon.
炭素14は炭素の放射性同位体です。
•
Scientists use different isotopes to date ancient artifacts.
科学者は古代の遺物を年代測定するために異なる同位体を使用します。